The Clinical Research Center
A Service of Retina Consultants of Minnesota
Dedicated to providing state-of-the-art treatment of eye diseases that cause vision loss by damaging the macula area of the retina.
For additional information please reach out to our clinical trial team.
St. Louis Park - 952-259-6264
Edina - 952-259-6262
About the Center
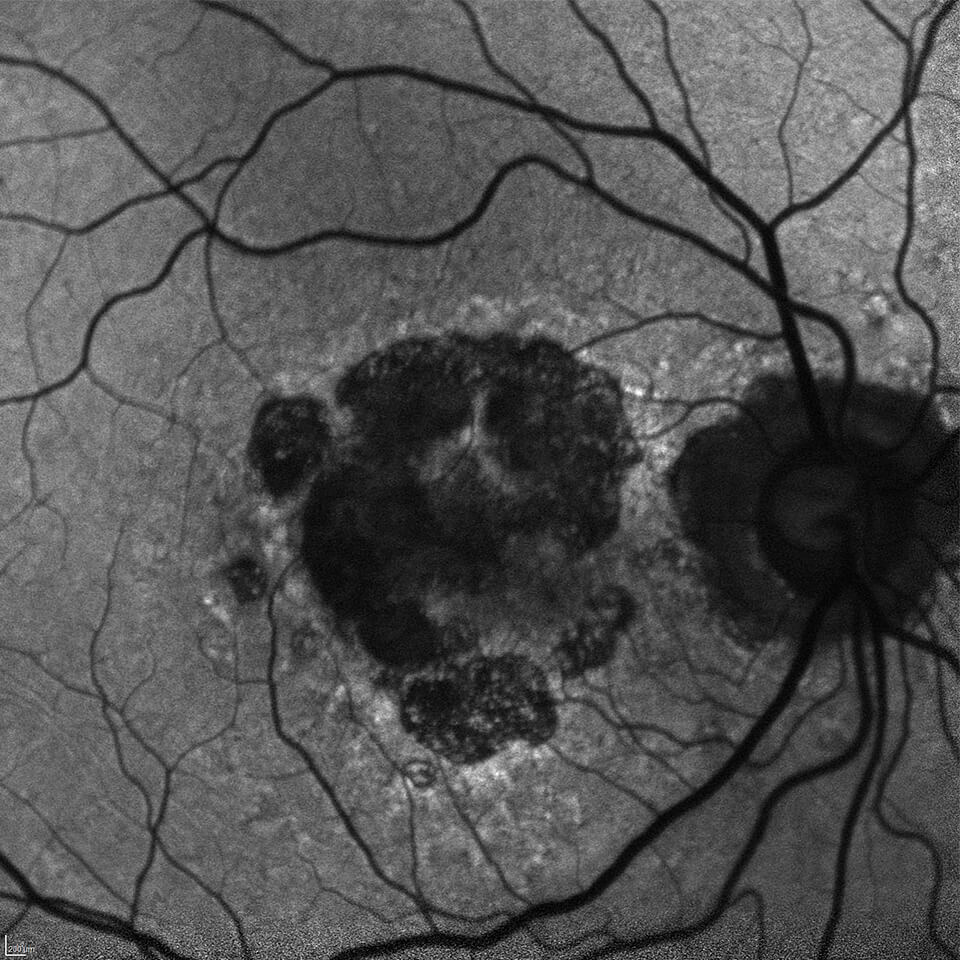
The Clinical Research Center is dedicated to advanced clinical research in the effort to find new and more effective treatments for Age-Related Macular Degeneration (AMD) and related diseases. We offer eligible patients who suffer from these diseases the opportunity to participate in a variety of clinical research trials that may offer access to advanced new treatments not yet available to the general public. A service of Retina Consultants of Minnesota (RCM), the Clinical Research Center is supported by fifteen Retina Specialists, a dedicated full-time clinical research staff, and the state-of-the-art retina treatment facilities of RCM.
Proven Experience
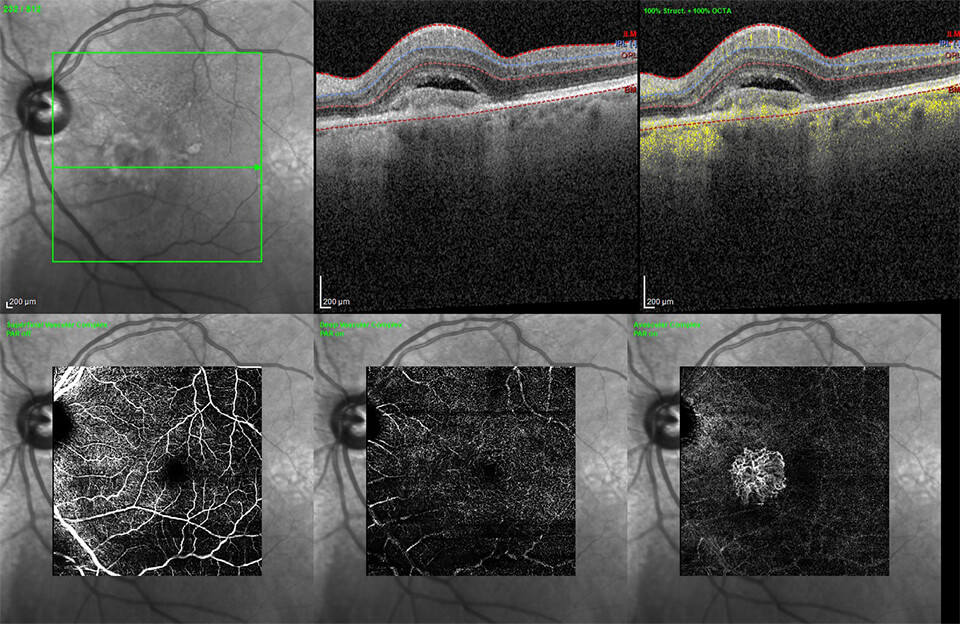
The Clinical Research Center is dedicated in fighting blindness through cutting-edge clinical research. We are one of the leading global centers committed to studying the vitreoretinal disorders.
Our dedicated research staff and advanced top of the line diagnostic imaging modalities and continues to pursue new advanced care to strengthen future care for patients with retinal disorders.
About Retina Consultants of Minnesota
The Clinical Research Center is the research division of Retina Consultants of Minnesota (RCM), one of the largest groups of physicians specializing in diseases of the macula, retina, and vitreous.
The physicians of RCM have over 200 years of cumulative experience in their field, have authored over 350 scientific articles, hold leadership positions in national and international professional organizations, and have been involved in research on the treatment of a variety of macular and retinal diseases since 1978.
Current Clinical Trials at RCM
Actively Enrolling
A PHASE IV, MULTICENTER, OPEN-LABEL, SINGLEARM STUDY OF THE RESPONSE TO TREATMENT AFTER TRANSITION TO THE PORT DELIVERY SYSTEM WITH RANIBIZUMAB (SUSVIMO [RANIBIZUMAB INJECTION]) IN PATIENTS WITH NEOVASCULAR AGE-RELATED MACULAR DEGENERATION PREVIOUSLY TREATED WITH INTRAVITREAL AGENTS OTHER THAN RANIBIZUMAB
A Randomized Clinical Trial Evaluating Fenofibrate for Prevention of Diabetic Retinopathy Worsening (Protocol AF)
A Phase VII study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of RO7446603 administered alone or in combination with Aflibercept or Faricimab in patients with diabetic macular edema
A Randomized, Double-masked, Sham-controlled, Multicenter, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal Tabirafusp Alfa (KSI-101) in Participants with Macular Edema Secondary to Inflammation (MESI) - PINNACLE
A Randomized, Double-masked, Sham-controlled, Multicenter, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal Tabirafusp Alfa (KSI-101) in Participants with Macular Edema Secondary to Inflammation (MESI) – PEAKA Phase 1b Open-label Study to Evaluate the Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Tinlarebant in Japanese Subjects with Stargardt Disease and a Phase 2/3 Randomized, Double-masked, and Placebo-controlled Study to Evaluate the Safety, Tolerability, and Efficacy of Tinlarebant in Subjects with Stargardt Disease
A non-interventional, observational study to evaluate treatment patterns and safety of avacincaptad pegol (ACP/IZERVAY™) in routine clinical practice in patients with geographic atrophy secondary to age-related macular degeneration
ORA SECUNDA CERCLAGE LASER RETINOPEXY TO PREVENT RETINAL DETACHMENT IN STICKLER SYNDROME (OSC/SS): A PROSPECTIVE HISTORICALLY CONTROLLED STUDY
Active Trials (Full)
A Single and Multiple Dose Study to Evaluate the Safety, Pharmacokinetics, and Treatment Effect of Intravitreal AVD-104 in Participants with Geographic Atrophy Secondary to Age-related Macular Degeneration
A Randomized, Double-Masked, Multi-Center, 3-Arm Pivotal Phase 2/3 Study to Evaluate The Efficacy and Safety of Intravitreal EYE103 Compared With Intravitreal Ranibizumab (0.5mg) in Participants With Diabetic Macular Edema
A Randomized, Double-masked, Multi-center, 3-Arm Pivotal Phase 2/3 Study to Evaluate the Efficacy and Safety of Intravitreal EYE103 Compared with Intravitreal ranibizumab (0.5mg) in Participants with Diabetic Macular Edema
A Three-Part, Phase I/II Study to Investigate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Zifibancimig Following Intravitreal Administration of Multiple Ascending Doses and Continuous Delivery From the Port Delivery in Patients With Neovascular Age-Related Macular Degeneration
A Study to Evaluate the Efficacy and Safety of Tarcocimab Tedromer and Tabirafusp Tedromer Compared to Aflibercept in Participants With Neovascular (Wet) Age-related Macular Degeneration (wAMD)
A Phase 3b, Single-Arm Study of Aflibercept 8 mg Dosed Every 4 Weeks in Adult Participants With Neovascular Age-Related Macular Degeneration (nAMD) or Diabetic Macular Edema (DME)
Phase II trial in patients with geographic atrophy: A randomized, double- masked, placebo-controlled, dose-finding study to evaluate the efficacy and safety of BI 1584862
A Phase I, multipart, multicenter study to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of Ro7497372
Following intravitreal administration in participants with diabetic macular edema (part 1 non-randomized, open-label, multiple ascending dose; part 2 randomized, double-masked)A Phase 3, Multicenter, Double-Masked, Randomized, Parallel-Group Study to Evaluate the Efficacy and Safety of Intravitreal OTX-TKI (axitinib implant) in Subjects with Neovascular Age-Related Macular Degeneration
A Randomized, Partially Masked, Controlled, Phase 3 Clinical Study to Evaluate the Efficacy and Safety of RGX-314 Gene Therapy in Participants with nAMD
A Randomized, Partially Masked, Controlled, Phase 2/3 Clinical Study to Evaluate the Efficacy and Safety of RGX-314 Gene Therapy in Participants with nAMD
Phase 4 PROSPECTIVE, MULTICENTER, OPEN-LABEL, OBSERVATIONAL PHASE 4 STUDY TO
EVALUATE REAL-WORLD SAFETY, TOLERABILITY, AND TREATMENT PATTERNS OF
PEGCETACOPLAN (SYFOVRE) IN PATIENTS WITH GEOGRAPHIC ATROPHY
SECONDARY TO AGE-RELATED MACULAR DEGENERATIONPHase 3, Multicenter, RandOmized, Double-masked, PlacEbo-CoNtrolled Study of TInlarebant to EXplore Safety and Efficacy in the Treatment of Geographic Atrophy
A Phase 3, Randomized, Double-Masked, Placebo-Controlled Clinical Trial to Evaluate the Efficacy, Safety, and Pharmacokinetics of Subcutaneous Injections of Elamipretide in Subjects who have Dry Age-Related Macular Degeneration (Dry AMD)
A Phase 3, Multicenter, Double-Masked, Randomized, Parallel-Group Study to Evaluate the Efficacy and Safety of Intravitreal OTX-TKI (Axitinib Implant) in Subjects with Neovascular Age-Related Macular Degeneration (nAMD).
Randomized, double-masked, active-controlled, multicenter study to evaluate efficacy and safety of two regimens of intravitreal BI 771716 against pegcetacoplan in participants with geographic atrophy secondary to age-related macular degeneration

